Scientists Develop Futuristic Material for Self-Healing Displays

Image via Konstantin Yolshin / Shutterstock
Toggle Dark Mode
Scientists have developed a new material that could lead to self-healing phones that repair themselves if scratched or cracked.
A team of researchers from the University of California Riverside have conducted a series of tests on the material, which is made of a stretchy polymer and an ionic salt — and it can be stretched to around 50 times its size. When they tore the substance in half, it automatically stitched itself back together in about a day, lead researcher Chao Wang said.
“Self-healing materials may seem very far away for real application, but I believe they will come out very soon with cellphones,” Wang told Business Insider. “Within three years, more self-healing products will go to market and change our everyday life. It will make our cellphones achieve much better performance than what they can achieve now.”
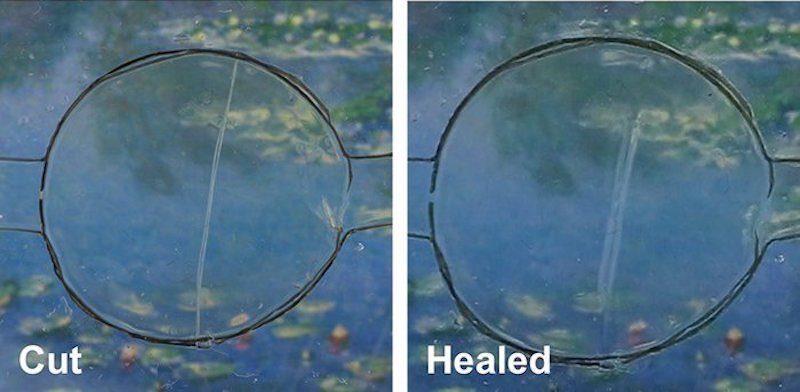
The polymer material’s self-healing properties rely on a special type of bond called an ion-dipole interaction, a “force between charged ions and polar molecules,” according to Science Daily. When torn, broken or scratched, this bond causes the ions and molecules of the material attract to each other — healing the damage. The UCR team’s material is also the first self-healing substance that is conductive, which is necessary for it to be used as a phone screen. While LG has included a self-healing material on the back covers of some of its phones, LG’s material can’t conduct electricity — making it useless for smartphone touchscreens.
The ramifications of such a material could be huge for the smartphone industry: it could lead to phone screens that automatically heal themselves from scratches and — potentially — cracks. It could lead to minor smartphone damage becoming a non-issue, and consequently, could make certain smartphone repairs irrelevant.
Wang said that the material could reach consumers by way of phone screens and batteries as early as 2020. The researchers will present the material and their research today at the 253rd National Meeting & Exposition of the American Chemical Society.






