Apple Watch Sleep Apnea Detection Gets Approved in Canada
Toggle Dark Mode
Earlier this month, Apple launched a Sleep Apnea detection feature in over 150 countries, from Afghanistan to the United States and beyond. However, the US’ northern neighbor was conspicuously missing from that list.
That’s because sleep apnea is one of those pesky medical features that has to be approved by health regulators in each country where Apple intends to launch it. In the United States, that’s the Food and Drug Administration (FDA), while Canada has its own health agency, with the rather obvious name Health Canada.
Even the FDA clearance came only days before watchOS 11 was set to go out to the public. It’s unclear what the process or timeline was in the other countries where sleep apnea detection launched, but Canada wasn’t alone in missing out on the feature at launch. While major markets like the United Kingdom, Japan, and most of the EU were on board, Australia and China also have yet to gain the feature (although Hong Kong is on the list).
Fortunately, Canadians shouldn’t have to wait much longer for the feature to arrive, as Health Canada has just published its approval for the feature, effective September 26, 2024. This joins previous approvals by the agency for the ECG app, irregular heart rhythm notifications, and Afib history.
Despite Health Canada’s approval, there’s no word on when Apple will unlock the Sleep Apnea feature for users in the Great White North and if a watchOS 11 point release will be required. For context, Health Canada approved the Apple Watch ECG feature on May 16, 2019, but it didn’t make it to the Apple Watch until watchOS 5.3 was released in July of that year.
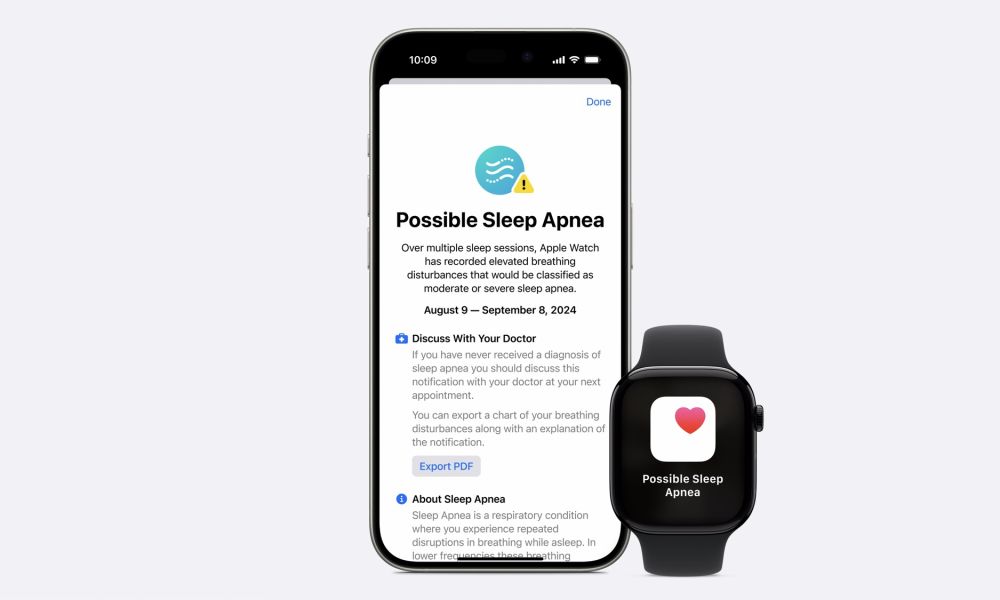
About Sleep Apnea Detection

Detecting and notifying Apple Watch users of sleep apnea was the marquee health feature in this year’s Apple Watch Series 10. However, Apple also surprised us by announcing that it would come to the Apple Watch Series 9 and Apple Watch Ultra 2 as part of watchOS 11.
Early rumors suggested the feature would rely on the blood oxygen sensor, which would have made things tricky since that’s currently banned in the US due to an ongoing patent dispute. Fortunately, Apple found another way.
As Apple’s VP of Health, Dr. Sumbal Desai explained at the company’s Glowtime event, the Apple Watch Series 9 and later (and Apple Watch Ultra 2) use a unique technique that relies on the accelerometer to analyze breathing and detect patterns typical of sleep apnea.
Apple outlined this further in a white paper, Estimating Breathing Disturbances and Sleep Apnea Risk from Apple Watch, which dives into the science and machine learning involved. It also outlines the clinical validation procedures Apple’s sleep apnea detection algorithms went through to ensure they worked reliably — and offers some insight into how complicated it truly is to get these kinds of health features ready for prime time.
The need for this kind of clinical study, testing, and validation is why health innovations on the Apple Watch have slowed down in recent years. Heart rate monitoring at the wrist is child’s play compared to more sophisticated things like blood oxygen, body temperature, blood pressure, sleep apnea, and blood glucose monitoring.
Apple has been working on some of these features for over a decade; for some, it hasn’t even bothered to seek medical certification. For example, the blood oxygen sensor is considered a “wellness” feature rather than a medical device, which allowed Apple to offer it without going through the FDA certification process.
That’s not an option for more sophisticated sensors like sleep apnea detection, and it’s unclear if Apple could offer hypertension alerts (simplified high blood pressure monitoring) similar to blood oxygen. Rumors suggested the company was planning to include hypertension in the Apple Watch Series 10 but shelved it during development. The reasons for that are unclear, but it’s likely Apple is still facing challenges getting it to work reliably.
Sources also suggest Apple may have finally cracked the nut on the holy grail of non-invasive blood glucose measurements. However, it still has a long road ahead to test and validate its findings to guarantee that it can deliver a feature that’s accurate and reliable. Earlier this year, the FDA warned users to avoid smartwatches and rings claiming to take these measurements. The agency emphasized that it hasn’t authorized or approved any such devices and that relying on them could harm users by resulting in improper use of medication used to manage diabetes.









