Apple Watch Gets FDA Greenlight for High Blood Pressure Alerts

Toggle Dark Mode
Apple’s recently announced hypertension detection feature for the Apple Watch has received FDA clearance and will launch next week, according to a 9to5Mac report.
Apple announced the new feature alongside the unveiling of the Apple Watch Series 11 and Apple Watch Ultra 3 on Tuesday. At the time, the Cupertino company said it was expecting the FDA to approve hypertension alerts “soon.” The FDA has now granted that clearance.
In addition to this year’s new Apple Watch Series 11 and Ultra 3 models, hypertension notifications will also be available on Apple Watch Series 9 and later, as well as the Apple Watch Ultra 2 and later models. However, this year’s Apple Watch SE 3 won’t be getting the feature, likely due to its use of the older second-generation optical heart sensor from the Apple Watch Series 5 era.
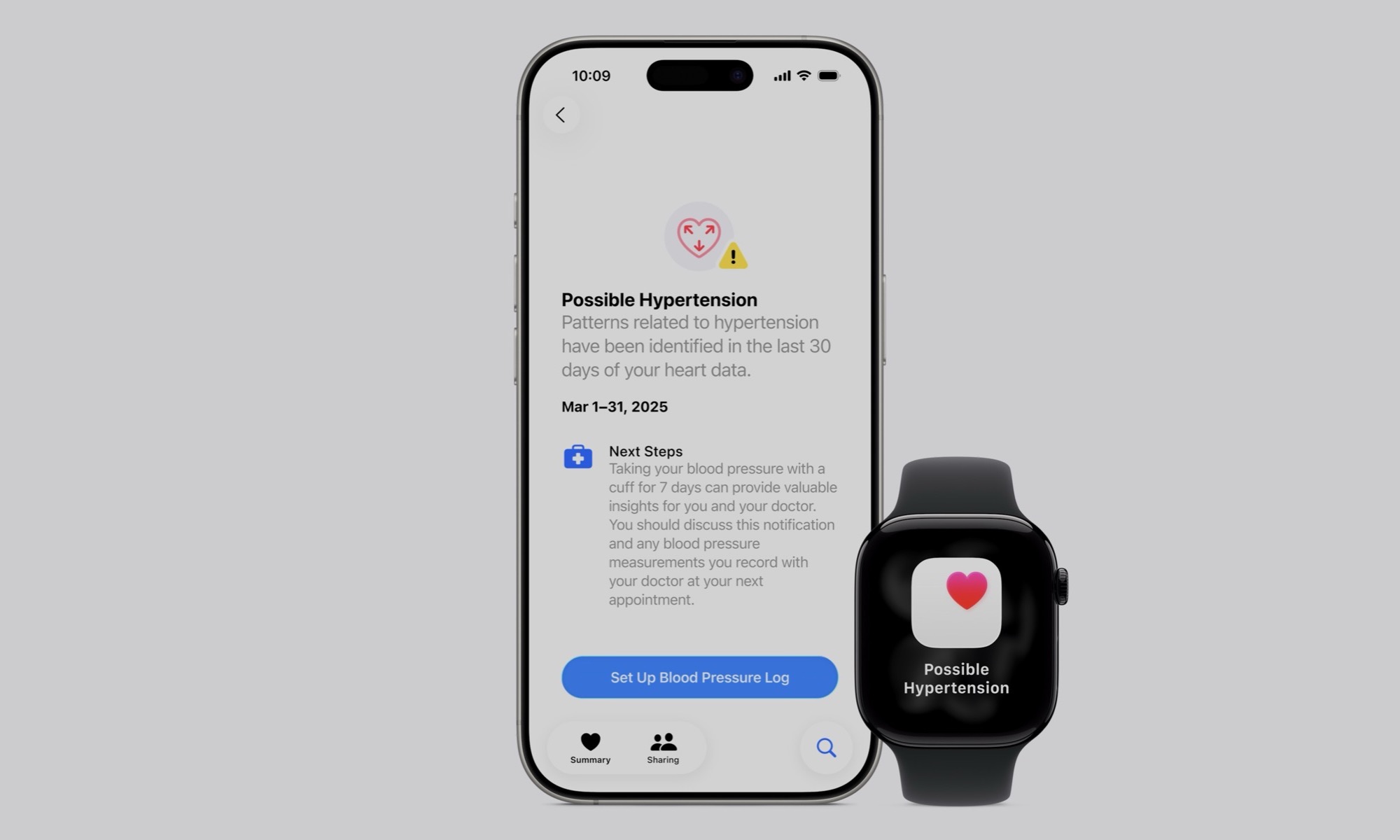
Hypertension notifications alert users if their Apple Watch detects signs of chronic high blood pressure (also known as hypertension). Apple notes that hypertension affects approximately 1.3 billion adults worldwide. The affliction is often misdiagnosed, as it often has no symptoms. Many affected individuals don’t see their doctor on a regular basis, and it can be easily missed by the single blood pressure measurement when making a visit to their health provider.
The feature uses data from the Apple Watch’s optical heart sensor, allowing it to analyze how the wearer’s blood vessels react to the beats of the user’s heart. The algorithm quietly collects data over 30-day periods and then notifies the user if consistent signs of hypertension are detected.
By simply wearing their Apple Watch, users receive valuable insights into their health, allowing them to make informed, potentially lifesaving decisions. This can lead to the user having discussions with their healthcare provider and possibly starting treatment to control what can lead to serious health issues.
Users who receive notifications are advised to monitor their blood pressure for seven days using a third-party cuff and share results with healthcare providers, which is consistent with the latest American Heart Association guidelines for the diagnosis and management of hypertension.
“Hypertension is the leading preventable cause of heart attack and stroke, yet millions remain undiagnosed,” said Harlan Krumholz, MD, SM, cardiologist and scientist at Yale University and Yale New Haven Hospital. “Making accurate detection easy and part of daily life can help people get care earlier and prevent avoidable harm.”
Apple says the hypertension notification feature is grounded in rigorous scientific validation. It was developed with advanced machine learning and training data from multiple studies totaling over 100,000 participants. The feature’s performance was subsequently validated in a clinical study involving over 2,000 participants. While the feature will not detect all forms of high blood pressure, it is expected to help over one million users detect undiagnosed high blood pressure.
Apple has said that the hypertension feature is ultimately expected to launch in the United States, the European Union, and 150+ other countries and regions this month. While it has now been cleared for the US, which means it should be available on supported US Apple Watch models when watchOS 26 rolls out on Monday, other countries may still be left waiting, as the feature requires approval from each country’s government health regulator.
For example, last year, the FDA approved the new sleep apnea detection feature on September 13, only days before watchOS 11 was released on September 16, giving Apple enough time to activate it in the US. However, the feature didn’t receive Health Canada approval until September 26, which meant Apple couldn’t include it for Canadians until the next scheduled software update arrived with watchOS 11.1 on October 24.
Apple will roll out watchOS 26 and iOS 26 on Monday, September 15. The new Apple Watch Series 11 and Apple Watch Ultra 3 are now available for preorder, starting at $399 and $799, respectively, and both will begin shipping on Friday, September 19.