AirPods Pro 2 Get FDA Approval for Use as Hearing Aids

Toggle Dark Mode
One of the few surprises at this week’s Glowtime event was the stage time given to Apple’s AirPods Pro. While their appearance provided a moment of hope that Apple might release the “AirPods Pro 3” sooner than expected, it turns out that the most significant change was coming to the software rather than the hardware.
Unlike last year’s AirPods Pro refresh that switched to a USB-C case with improved dust resistance, this year’s hardware hasn’t changed. However, in addition to the cool new iOS 18 features we already heard about at June’s Worldwide Developers Conference (WWDC), Apple had another big surprise: it’s turning the AirPods Pro into a powerful way to improve your hearing health.
That should have been apparent when Apple’s VP of Health, Dr. Sumbal Desai, took the stage to introduce them. Dr. Desai noted that more than 1.5 billion people live with hearing loss, which results in an increased risk of cognitive decline, social isolation, and depression for many. As a result, Apple decided it was time to do what it could to help with the AirPods Pro.

Apple is tackling this in three areas, first by improving its prevention techniques. In addition to existing methods such as the Noise app and hearing health tracking, the AirPods Pro will now have hearing protection enabled by default to minimize exposure to loud noises. The ear tips offer passive noise isolation, while the H2 chip powers machine learning algorithms that can reduce louder intermittent noises to keep them from damaging your eardrums.
Since 75% of people with hearing loss are untreated and haven’t received any assistance — and many may not even be aware of a slow decline in their hearing — Apple is also adding new features to increase awareness. This includes a clinically validated Hearing Test that can be taken in the comfort of your home to help you identify if you have a hearing problem.
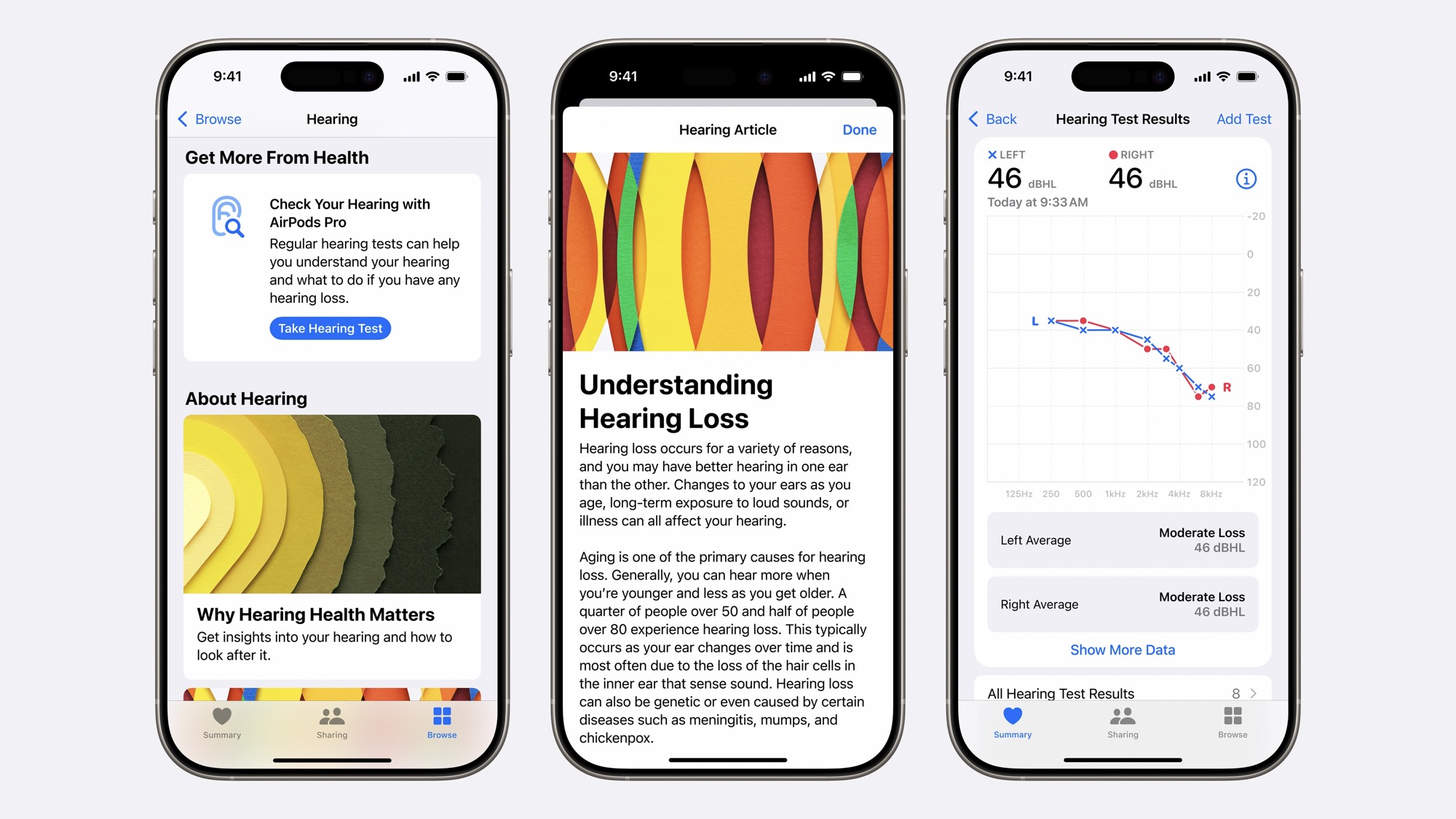
However, the AirPods Pro will go a big step beyond that to help you with any hearing problems as much as possible. Thanks to new FDA regulations permitting over-the-counter hearing aids, the AirPods Pro can automatically fill that gap based on your hearing test results. This not only recommends that your AirPods Pro be used as a hearing aid, but it also tunes them with a profile unique to your situation. Plus, that profile will be automatically applied to what you listen to through your AirPods Pro, such as music, movies, podcasts, and TV shows.
When Dr. Desai announced the new features, she said they’d be coming in a software update to the AirPods and iOS 18 once Apple receives clearance from the US Food and Drug Administration (FDA), which was expected to happen “soon.”
It turns out she wasn’t exaggerating at all in her use of the word. The FDA officially authorized the AirPods Pro update on Thursday, citing it as the “first over-the-counter hearing aid software.”
Hearing loss is a significant public health issue impacting millions of Americans. Today’s marketing authorization of an over-the-counter hearing aid software on a widely used consumer audio product is another step that advances the availability, accessibility and acceptability of hearing support for adults with perceived mild to moderate hearing loss.Michelle Tarver, FDA
In the FDA’s press release, the agency notes that the hearing aid feature (HAF) was “evaluated in a clinical study with 118 subjects with perceived mild to moderate hearing loss, at multiple U.S. sites,” which showed that users fitting their AirPods Pro through the guided steps int he iPhone Health app “achieved similar perceived benefit” as those having them professionally fitted.
With the FDA’s green light, the ball is back in Apple’s court to get the necessary software updates pushed out. There’s a slight possibility these could be enabled in time for the launch of iOS 18.0 on Monday, especially if Apple had merely switched them off pending FDA clearance. However, it may still take a bit longer to get everything ready, especially since Apple plans to release these features in over 100 countries, and they’re still pending marketing authorization from other global health authorities.
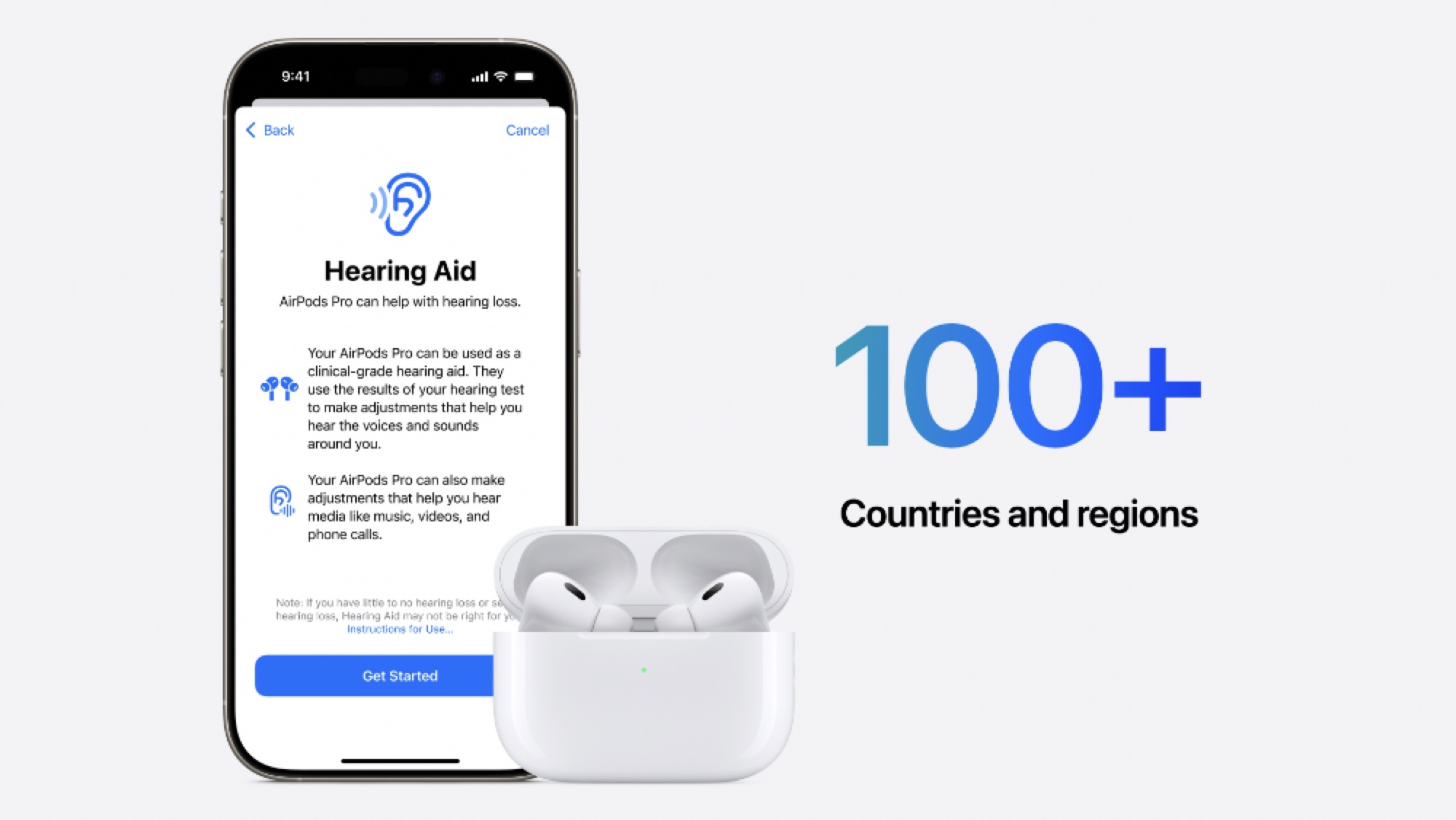
There are also countries and regions where not all hearing health features will be available. For example, as iPhone in Canada reported earlier this week, Canucks may have to wait for the Hearing Aid feature as Health Canada doesn’t have an equivalent to the FDA’s approval of over-the-counter (OTC) hearing aids — they still have to be prescribed by a licensed audiologist or other hearing professional.







